

The elements of the first three periods of the periodic table are shown below: We can infer how the electrons are arranged around a nucleus by considering the position of an element in the periodic table.

Once both the 1 st energy level (K shell) and the second energy level (L shell) are full, we must begin filling the next energy level, the 3 rd energy level (M shell). until the 2 nd energy level (L shell) is filled with 8 electrons. (2 electrons in the first energy level and 1 electron in the second energy level)įor atoms with more than 3 electrons, we can continue adding electrons to the second energy level or L shell until it reaches its maximum capacity of 8 electrons:įourth electron goes into the 2 nd energy level (L shell). The electron configuration (electronic configuration) of the atom is 2,1 So instead of talking about "shells" we can also talk about energy levels:Īnd now we can draw the energy levels only, without drawing the rest of the circle to represent the shell: So, scientists have come up with a short cut for representing the electronic structure of atoms, and this is based on an understanding of the energy of electrons in each shell.Įlectrons closest to the nucleus, in the K shell, have less energy than electrons in the next shell out from the nucleus, the L shell.Įnergy of electrons increases as they get further away from the nucleus. We could draw the electronic structure of each atom by placing a dot representing an electron in each shell, but that would take up a lot of space. Indeed, we find that the K shell can contain a maximum of only 2 electrons, but the next shell, the L shell, can contain a maximum of 8 electrons. The shell closest to the nucleus is given the symbol K, the next shell is the L shell, which is followed by the M shell, then the N shell:Īs the boundary of each shell gets further from the nucleus, the shell gets larger, so it makes sense that shells closer to the nucleus can contain fewer electrons than shells further away from the nucleus. In the diagram below, the black circle represents the nucleus of the atom, and each circle represents the boundary of an electron shell.
#FULL CARBON ELECTRON CONFIGURATION SERIES#
We can model the electronic structure of atoms as a series of concentric spheres, or shells, around the nucleus of an atom. protons = atomic number (Z)īut the atomic number (Z) only tells us how many electrons in total there are around the nucleus of a neutral atom, it doesn't directly tell us how those electrons are arranged around the nucleus. Therefore, the number of electrons in an atom of an element is equal to the element's atomic number (Z):
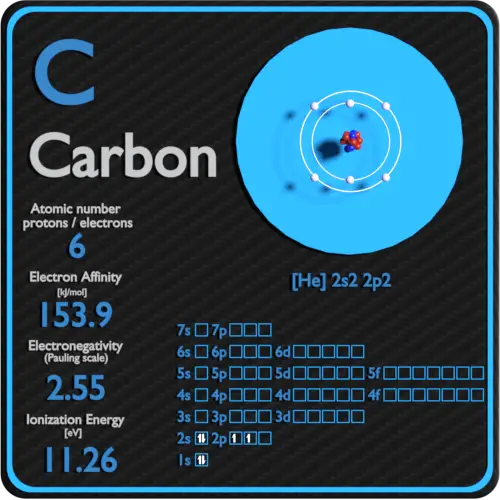
The number of protons in the nucleus of an atom is equal to its atomic number (Z). That is, for an atom to be neutral (no overall charge), the number of negative charges (electrons) must be equal to the number of positive charges (protons). The number of electrons around the nucleus of each atom is equal to the number of protons in its nucleus.
#FULL CARBON ELECTRON CONFIGURATION FREE#
No ads = no money for us = no free stuff for you! Electron Shell Concept Electron configurations (electronic configurations) are written with number of electrons in lowest energy level (shell) first, separated by a comma from the number of electrons in the next energy level (shell), etc.L shell (2 nd energy level) can hold a maximum of 8 electrons. K shell (1 st energy level) can hold a maximum of 2 electrons.Electrons fill the lowest energy shells (energy levels) first.N is the 4 th energy level (higher energy than M shell) M is the 3 rd energy level (higher energy than L shell)
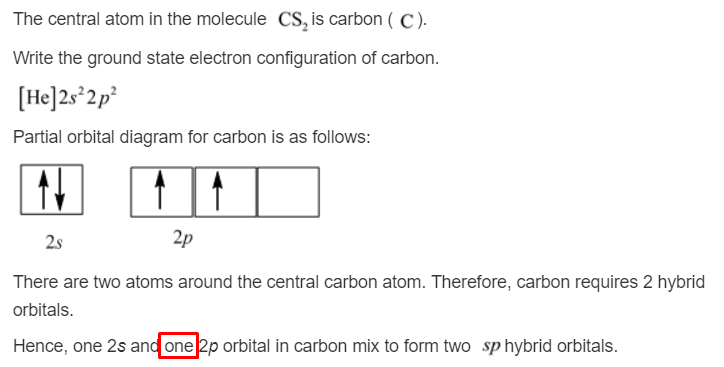
L is the 2 nd energy level (higher energy than K shell) K is the 1 st energy level (lowest energy level) Electron shells correspond to energy levels:.Shells in order from closest to furthest from nucleus:.Shells are given the symbols K, L, M and N.An atom is represented as a nucleus surrounded by shells containing electrons.You need to become an AUS-e-TUTE Member! Periods 1 to 3 Atoms: Electron Configuration in Shells (energy levels) Chemistry Tutorial Key Concepts Want chemistry games, drills, tests and more? Shell Electron Configuration Periods 1 to 3 Atoms Chemistry Tutorial More Free Tutorials Become a Member Members Log‐in Contact Us


 0 kommentar(er)
0 kommentar(er)
